Brain Imaging
What if we could use tools to diagnose Alzheimer’s disease and dementia before the symptoms of cognitive and mental deterioration became clinically apparent?
The future hope is to intervene with targeted therapies to treat the disease in its earliest stages, before irreversible brain cell loss and cognitive decline occur.
Research on new strategies for earlier diagnosis has focused on the potential role of neuroimaging as one tool to help aid in the earlier detection and diagnosis of Alzheimer’s disease and related dementias.
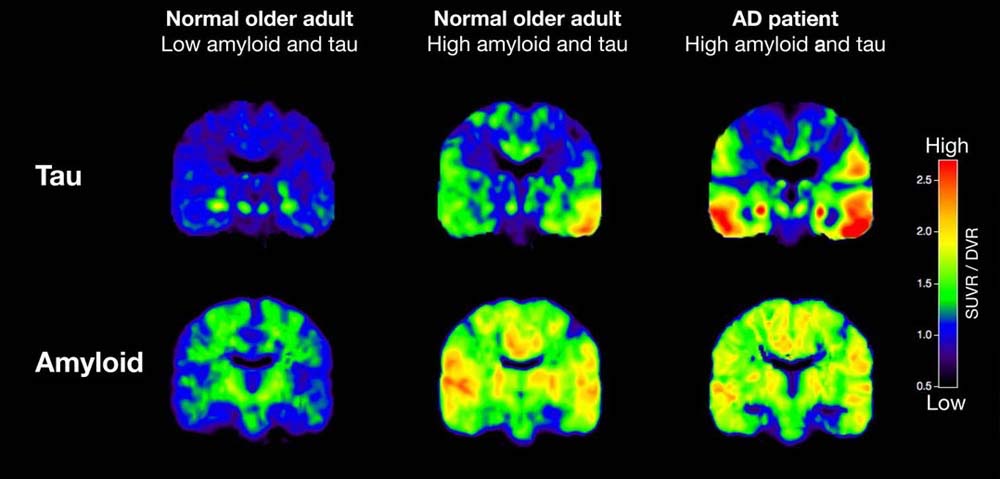
Amyloid and Tau Imaging/Source PsyPost
Other brain imaging techniques under study such as amyloid and tau imaging may be useful as a possible technique for enhanced diagnosis and tracking of the progress of Alzheimer’s disease.
Brain Imaging Types
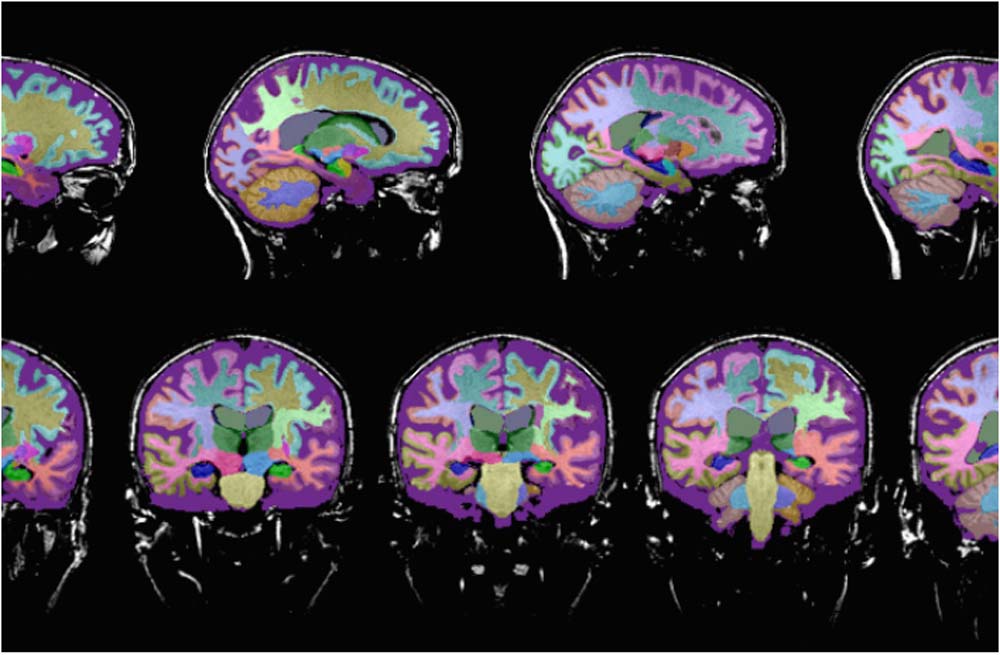
Neuroimaging is a critical tool for assessing the physical, structural and functional brain changes that occur in different parts of the brain during various neurodegenerative conditions.
- Structural imaging provides information about the shape, location and volume of brain tissue. Structural imaging techniques include computer tomography (CT) and magnetic resonance imaging (MRI).
- Functional imaging reveals the extent to which cells in different regions of the brain are performing by assessing how well the cells use sugar (glucose) or oxygen. Functional techniques include positron emission tomography (PET) and functional MRI (fMRI).
- Molecular imaging uses short-lived radioactive tracer compounds to detect cellular or chemical changes in the brain that are associated with various diseases. Molecular imaging technologies include PET, fMRI and single photon emission computed tomography (SPECT).
These imaging techniques can be helpful in the diagnosis of conditions such as Alzheimer’s disease (AD), frontotemporal dementia, amyotrophic lateral sclerosis with and without dementia, Parkinson’s disease with and without dementia, dementia with Lewy Bodies, Huntington’s disease, multiple sclerosis, HIV-associated neurocognitive disorder, and prion protein associated diseases (i.e., Creutzfeldt-Jakob disease) among other conditions.
However, we do not yet have enough information to translate these patterns of structural changes or reduced brain activity into definitive diagnostic information about individuals. These tools must be used together with other diagnostics techniques, such as neuropsychological evaluation and laboratory/serum blood tests, to arrive at a definitive diagnosis.
Brain Imaging in the diagnosis Alzheimer’s disease or other dementias
Currently, the standard medical work-up for Alzheimer’s disease includes structural imaging with magnetic resonance imaging (MRI) or computed tomography (CT). These tests are mainly used to rule out other conditions that may cause similar symptoms to Alzheimer’s disease but require different interventional strategies or treatments.
Structural imaging can reveal tumors, areas of vascular damage or stroke, damage from prior significant traumatic brain injury or even a buildup of fluid in the brain as found in normal pressure hydrocephalus. Structural imaging studies may also reveal shrinkage (atrophy) in the brains of people with Alzheimer’s disease that worsens as the disease progresses.
Neuroreader™ is a computerized program that provides an assessment of total brain volume, hippocampal volume and volumetric data on different anatomical areas of the brain measured against a healthy database.
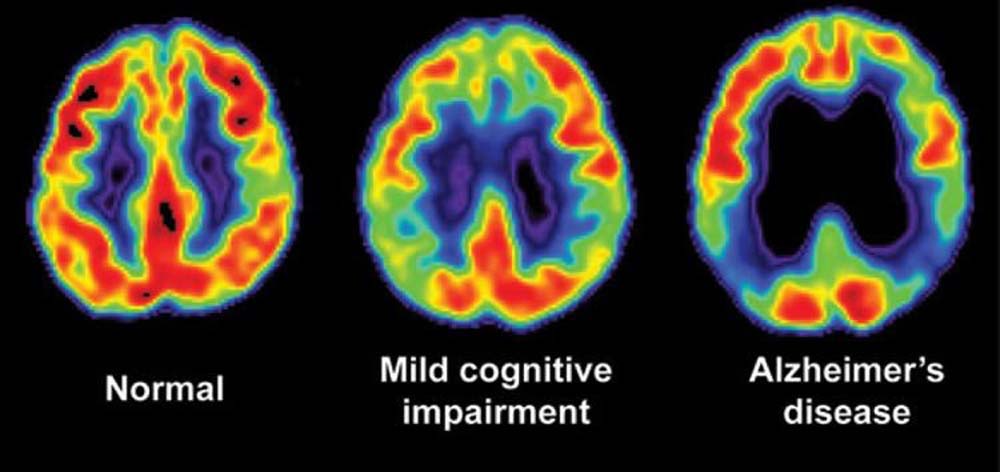
Fluorodeoxyglucose (FDG)-PET scanning in patients with Alzheimer’s disease shows reduced use of glucose (sugar) in areas of the brain that are important in memory, learning and problem-solving.
Molecular imaging tracers. In 2012, the U.S. Food and Drug Administration approved the first molecular imaging tracer for use in patients under assessment for possible Alzheimer’s disease or other causes of cognitive deterioration. This tracer (brand name Amyvid but also known as florbetapir F-18), is a radioactive tracer molecule that binds to beta-amyloid, one of the signature protein deposits found in the brains of patients with Alzheimer’s disease.
Because it is labeled with a small amount of radioactive tracer it can be visualized during a positron emission tomography (PET) brain scan, thereby revealing the presence of amyloid plaques in the brains of living patients.
Other, newer molecular imaging tracers have also been developed that also help to reveals amyloid plaques in the brain during PET imaging. For example, Vizamyl, also known as flutametamol F18 was approved in 2013 and Neuraceq, also known as florbetaben F18 was approved in 2014.
Even though amyloid plaques in the brain are a characteristic feature of Alzheimer’s disease, their presence is not definitive proof of the presence of the disease. Healthy elderly people may have amyloid plaque deposits in the brain without symptoms of cognitive decline or Alzheimer’s disease. Because amyloid plaques cannot be used to diagnose Alzheimer’s disease, amyloid imaging is not recommended for routine use in patients suspected of having Alzheimer’s disease.
A Task Force of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the Alzheimer’s Association has published criteria that outline situations in which the use of amyloid imaging would be appropriate.